Mexitil Prescribing Information
Package insert / product label
Generic name: mexiletine hydrochloride
Dosage form: Capsules
Drug class: Group I antiarrhythmics
Medically reviewed by Drugs.com. Last updated on Mar 25, 2024.
The Mexitil brand name has been discontinued in the U.S. If generic versions of this product have been approved by the FDA, there may be generic equivalents available.
On This Page
WARNINGS
Mortality: In the National Heart, Lung and Blood Institute's Cardiac Arrhythmia Suppression Trial (CAST), a long-term, multicentered, randomized, double-blind study in patients with asymptomatic non-life-threatening ventricular arrhythmias who had a myocardial infarction more than six days but less than two years previously, an excessive mortality or non-fatal cardiac arrest rate (7.7%) was seen in patients treated with encainide or flecainide compared with that seen in patients assigned to carefully matched placebo-treated groups (3.0%). The average duration of treatment with encainide or flecainide in this study was ten months.
The applicability of the CAST results to other populations (e.g., those without recent myocardial infarction) is uncertain. Considering the known proarrhythmic properties of MEXITIL and the lack of evidence of improved survival for any antiarrhythmic drug in patients without life-threatening arrhythmias, the use of MEXITIL as well as other antiarrhythmic agents should be reserved for patients with life-threatening ventricular arrhythmia.
Mexitil Description
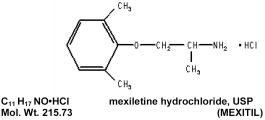
MEXITIL® (mexiletine hydrochloride, USP) is an orally active antiarrhythmic agent available as 150 mg, 200 mg and 250 mg capsules. 100 mg of mexiletine hydrochloride is equivalent to 83.31 mg of mexiletine base. It is a white to off-white crystalline powder with slightly bitter taste, freely soluble in water and in alcohol. MEXITIL has a pKa of 9.2.
Chemically, MEXITIL is 1-methyl-2-(2, 6-xylyloxy) ethylamine hydrochloride and has the following structural formula:
MEXITIL capsules contain the following excipients: colloidal silicon dioxide, corn starch, magnesium stearate, titanium dioxide, gelatin, pharmaceutical glaze, simethicone, FD&C Red No. 40, and FD&C Blue No. 1; the MEXITIL 150 mg and 250 mg capsules also contain FD&C Yellow No. 10 and D&C Red No. 28. MEXITIL capsules may contain one or more of the following components: sodium lauryl sulfate, lecithin, shellac, and FD&C Blue No. 1 Aluminum Lake.
Mexitil - Clinical Pharmacology
Mechanism of Action
MEXITIL (mexiletine hydrochloride, USP) is a local anesthetic, antiarrhythmic agent, structurally similar to lidocaine, but orally active. In animal studies, MEXITIL has been shown to be effective in the suppression of induced ventricular arrhythmias, including those induced by glycoside toxicity and coronary artery ligation. MEXITIL, like lidocaine, inhibits the inward sodium current, thus reducing the rate of rise of the action potential, Phase 0. MEXITIL decreased the effective refractory period (ERP) in Purkinje fibers. The decrease in ERP was of lesser magnitude than the decrease in action potential duration (APD), with a resulting increase in the ERP/APD ratio.
Electrophysiology in Man
Mexiletine is a Class 1B antiarrhythmic compound with electrophysiologic properties in man similar to lidocaine, but dissimilar from quinidine, procainamide, and disopyramide.
In patients with normal conduction systems, MEXITIL has a minimal effect on cardiac impulse generation and propagation. In clinical trials, no development of second-degree or third-degree AV block was observed. MEXITIL did not prolong ventricular depolarization (QRS duration) or repolarization (QT intervals) as measured by electrocardiography. Theoretically, therefore, MEXITIL may be useful in the treatment of ventricular arrhythmias associated with a prolonged QT interval.
In patients with pre-existing conduction defects, depression of the sinus rate, prolongation of sinus node recovery time, decreased conduction velocity and increased effective refractory period of the intraventricular conduction system have occasionally been observed.
The antiarrhythmic effect of MEXITIL has been established in controlled comparative trials against placebo, quinidine, procainamide and disopyramide. MEXITIL, at doses of 200-400 mg q8h, produced a significant reduction of ventricular premature beats, paired beats, and episodes of non-sustained ventricular tachycardia compared to placebo and was similar in effectiveness to the active agents. Among all patients entered into the studies, about 30% in each treatment group had a 70% or greater reduction in PVC count and about 40% failed to complete the 3 month studies because of adverse effects. Follow-up of patients from the controlled trials has demonstrated continued effectiveness of MEXITIL in long-term use.
Hemodynamics
Hemodynamic studies in a limited number of patients, with normal or abnormal myocardial function, following oral administration of MEXITIL, have shown small, usually not statistically significant, decreases in cardiac output and increases in systemic vascular resistance, but no significant negative inotropic effect. Blood pressure and pulse rate remain essentially unchanged. Mild depression of myocardial function, similar to that produced by lidocaine, has occasionally been observed following intravenous MEXITIL therapy in patients with cardiac disease.
Pharmacokinetics
MEXITIL is well absorbed (~90%) from the gastrointestinal tract. Unlike lidocaine, its first-pass metabolism is low. Peak blood levels are reached in two to three hours. In normal subjects, the plasma elimination half-life of MEXITIL is approximately 10-12 hours. It is 50-60% bound to plasma protein, with a volume of distribution of 5-7 liters/kg. MEXITIL is mainly metabolized in the liver, the primary pathway being CYP2D6 metabolism, although it is also a substrate for CYP1A2. With involvement of CYP2D6, there can be either poor or extensive metabolizer phenotypes. Since approximately 90% of MEXITIL is metabolized in the liver into inactive metabolites, pathological changes in the liver can restrict hepatic clearance of MEXITIL and its metabolites. The metabolic degradation proceeds via various pathways including aromatic and aliphatic hydroxylation, dealkylation, deamination and N-oxidation. Several of the resulting metabolites are submitted to further conjugation with glucuronic acid (phase II metabolism); among these are the major metabolites p-hydroxymexiletine, hydroxy-methylmexiletine and N-hydroxy-mexiletine. Approximately 10% is excreted unchanged by the kidney. While urinary pH does not normally have much influence on elimination, marked changes in urinary pH influence the rate of excretion: acidification accelerates excretion, while alkalinization retards it.
Several metabolites of mexiletine have shown minimal antiarrhythmic activity in animal models. The most active is the minor metabolite N-methylmexiletine, which is less than 20% as potent as mexiletine. The urinary excretion of N-methylmexiletine in man is less than 0.5%. Thus the therapeutic activity of MEXITIL is due to the parent compound.
Hepatic impairment prolongs the elimination half-life of MEXITIL. In eight patients with moderate to severe liver disease, the mean half-life was approximately 25 hours.
Consistent with the limited renal elimination of MEXITIL, little change in the half-life has been detected in patients with reduced renal function. In eight patients with creatinine clearance less than 10 ml/min, the mean plasma elimination half-life was 15.7 hours; in seven patients with creatinine clearance between 11-40 ml/min, the mean half-life was 13.4 hours.
The absorption rate of MEXITIL is reduced in clinical situations such as acute myocardial infarction in which gastric emptying time is increased. Narcotics, atropine and magnesium-aluminum hydroxide have also been reported to slow the absorption of MEXITIL. Metoclopramide has been reported to accelerate absorption.
Mexiletine plasma levels of at least 0.5 mcg/ml are generally required for therapeutic response. An increase in the frequency of central nervous system adverse effects has been observed when plasma levels exceed 2.0 mcg/ml. Thus the therapeutic range is approximately 0.5 to 2.0 mcg/ml. Plasma levels within the therapeutic range can be attained with either three times daily or twice daily dosing but peak to trough differences are greater with the latter regimen, creating the possibility of adverse effects at peak and arrhythmic escape at trough. Nevertheless, some patients may be transferred successfully to the twice daily regimen. (See DOSAGE AND ADMINISTRATION).
Related/similar drugs
amiodarone, lidocaine, verapamil, flecainide, propafenone, Pacerone, dofetilide
Indications and Usage for Mexitil
MEXITIL (mexiletine hydrochloride, USP)is indicated for the treatment of documented ventricular arrhythmias, such as sustained ventricular tachycardia, that, in the judgement of the physician, are life-threatening. Because of the proarrhythmic effects of MEXITIL, its use with lesser arrhythmias is generally not recommended. Treatment of patients with asymptomatic ventricular premature contractions should be avoided.
Initiation of MEXITIL treatment, as with other antiarrhythmic agents used to treat life-threatening arrhythmias, should be carried out in the hospital.
Antiarrhythmic drugs have not been shown to enhance survival in patients with ventricular arrhythmias.
Contraindications
MEXITIL (mexiletine hydrochloride, USP) is contraindicated in the presence of cardiogenic shock or pre-existing second- or third-degree AV block (if no pacemaker is present).
Acute Liver Injury
In post-marketing experience abnormal liver function tests have been reported, some in the first few weeks of therapy with MEXITIL (mexiletine hydrochloride, USP). Most of these have been observed in the setting of congestive heart failure or ischemia and their relationship to MEXITIL has not been established.
Precautions
General
If a ventricular pacemaker is operative, patients with second or third degree heart block may be treated with MEXITIL (mexiletine hydrochloride, USP) if continuously monitored. A limited number of patients (45 of 475 in controlled clinical trials) with pre-existing first degree AV block were treated with MEXITIL; none of these patients developed second or third degree AV block. Caution should be exercised when it is used in such patients or in patients with pre-existing sinus node dysfunction or intraventricular conduction abnormalities.
Like other antiarrhythmics MEXITIL (mexiletine hydrochloride, USP) can cause worsening of arrhythmias. This has been uncommon in patients with less serious arrhythmias (frequent premature beats or non-sustained ventricular tachycardia: see ADVERSE REACTIONS), but is of greater concern in patients with life-threatening arrhythmias such as sustained ventricular tachycardia. In patients with such arrhythmias subjected to programmed electrical stimulation or to exercise provocation, 10-15% of patients had exacerbation of the arrhythmia, a rate not greater than that of other agents.
MEXITIL should be used with caution in patients with hypotension and severe congestive heart failure because of the potential for aggravating these conditions.
Since MEXITIL is metabolized in the liver, and hepatic impairment has been reported to prolong the elimination half-life of MEXITIL, patients with liver disease should be followed carefully while receiving MEXITIL. The same caution should be observed in patients with hepatic dysfunction secondary to congestive heart failure.
Concurrent drug therapy or dietary regimens which may markedly alter urinary pH should be avoided during MEXITIL therapy. The minor fluctuations in urinary pH associated with normal diet do not affect the excretion of MEXITIL.
SGOT Elevation and Liver Injury
In three-month controlled trials, elevations of SGOT greater than three times the upper limit of normal occurred in about 1% of both mexiletine-treated and control patients. Approximately 2% of patients in the mexiletine compassionate use program had elevations of SGOT greater than or equal to three times the upper limit of normal. These elevations frequently occurred in association with identifiable clinical events and therapeutic measures such as congestive heart failure, acute myocardial infarction, blood transfusions and other medications. These elevations were often asymptomatic and transient, usually not associated with elevated bilirubin levels and usually did not require discontinuation of therapy. Marked elevations of SGOT (>1000 U/L) were seen before death in four patients with end-stage cardiac disease (severe congestive heart failure, cardiogenic shock).
Rare instances of severe liver injury, including hepatic necrosis, have been reported in association with MEXITIL treatment. It is recommended that patients in whom an abnormal liver test has occurred, or who have signs or symptoms suggesting liver dysfunction, be carefully evaluated. If persistent or worsening elevation of hepatic enzymes is detected, consideration should be given to discontinuing therapy.
Blood Dyscrasias
Among 10,867 patients treated with mexiletine in the compassionate use program, marked leukopenia (neutrophils less than 1000/mm3) or agranulocytosis were seen in 0.06% and milder depressions of leukocytes were seen in 0.08%, and thrombocytopenia was observed in 0.16%. Many of these patients were seriously ill and receiving concomitant medications with known hematologic adverse effects. Rechallenge with mexiletine in several cases was negative. Marked leukopenia or agranulocytosis did not occur in any patient receiving MEXITIL alone; five of the six cases of agranulocytosis were associated with procainamide (sustained release preparations in four) and one with vinblastine. If significant hematologic changes are observed, the patient should be carefully evaluated, and, if warranted, MEXITIL should be discontinued. Blood counts usually return to normal within one month of discontinuation. (See ADVERSE REACTIONS).
Convulsions (seizures) did not occur in MEXITIL controlled clinical trials. In the compassionate use program, convulsions were reported in about 2 of 1000 patients. Twenty-eight percent of these patients discontinued therapy. Convulsions were reported in patients with and without a prior history of seizures. Mexiletine should be used with caution in patients with known seizure disorder.
Drug Interactions
Since MEXITIL is a substrate for the metabolic pathways involving CYP2D6 and CYP1A2 enzymes, inhibition or induction of either of these enzymes would be expected to alter mexiletine plasma concentrations. In a formal, single-dose interaction study (n = 6 males) the clearance of mexiletine was decreased by 38% following the coadministration of fluvoxamine, an inhibitor of CYP1A2. In another formal study (n = 8 extensive and n = 7 poor metabolizers of CYP2D6), coadministration of propafenone did not alter the kinetics of mexiletine in the poor CYP2D6 metabolizer group. However, the metabolic clearance of mexiletine in the extensive metabolizer phenotype decreased by about 70% making the poor and extensive metabolizer groups indistinguishable. In this crossover steady state study, the pharmacokinetics of propafenone were unaffected in either phenotype by the coadministration of mexiletine. Addition of mexiletine to propafenone did not lead to further electrocardiographic parameters changes of QRS, QTc, RR, and PR intervals than propafenone alone. When concomitant administration of either of these two drugs with mexiletine is initiated, the dose of mexiletine should be slowly titrated to desired effect.
In a large compassionate use program MEXITIL has been used concurrently with commonly employed antianginal, antihypertensive, and anticoagulant drugs without observed interactions. A variety of antiarrhythmics such as quinidine or propranolol were also added, sometimes with improved control of ventricular ectopy. When phenytoin or other hepatic enzyme inducers such as rifampin and phenobarbital have been taken concurrently with MEXITIL®, lowered MEXITIL plasma levels have been reported. Monitoring of MEXITIL plasma levels is recommended during such concurrent use to avoid ineffective therapy.
In a formal study, benzodiazepines were shown not to affect MEXITIL plasma concentrations. ECG intervals (PR, QRS, and QT) were not affected by concurrent MEXITIL and digoxin, diuretics, or propranolol.
Concurrent administration of cimetidine and MEXITIL has been reported to increase, decrease, or leave unchanged MEXITIL plasma levels; therefore patients should be followed carefully during concurrent therapy.
MEXITIL does not alter serum digoxin levels but magnesium-aluminum hydroxide, when used to treat gastrointestinal symptoms due to MEXITIL (mexiletine hydrochloride, USP) Capsules, has been reported to lower serum digoxin levels.
Concurrent use of MEXITIL and theophylline may lead to increased plasma theophylline levels. One controlled study in eight normal subjects showed a 72% mean increase (range 35-136%) in plasma theophylline levels. This increase was observed at the first test point which was the second day after starting MEXITIL. Theophylline plasma levels returned to pre- MEXITIL values within 48 hours after discontinuing MEXITIL. If MEXITIL and theophylline are to be used concurrently, theophylline blood levels should be monitored, particularly when the MEXITIL dose is changed. An appropriate adjustment in theophylline dose should be considered.
Additionally, in one controlled study in five normal subjects and seven patients, the clearance of caffeine was decreased 50% following the administration of MEXITIL.
Carcinogenesis, Mutagenesis and Impairment of Fertility
Studies of carcinogenesis in rats (24 months) and mice (18 months) did not demonstrate any tumorigenic potential. MEXITIL was found to be non-mutagenic in the Ames test. MEXITIL did not impair fertility in the rat.
Pregnancy/Teratogenic Effects PREGNANCY CATEGORY C
Reproduction studies performed with MEXITIL (mexiletine hydrochloride, USP) in rats, mice and rabbits at doses up to four times the maximum human oral dose (24 mg/kg in a 50 kg patient) revealed no evidence of teratogenicity or impaired fertility but did show an increase in fetal resorption. There are no adequate and well-controlled studies in pregnant women; this drug should be used in pregnancy only if the potential benefit justifies the potential risk to the fetus.
Adverse Reactions/Side Effects
MEXITIL (mexiletine hydrochloride, USP) commonly produces reversible gastrointestinal and nervous system adverse reactions but is otherwise well tolerated. MEXITIL has been evaluated in 483 patients in one-month and three-month controlled studies and in over 10,000 patients in a large compassionate use program. Dosages in the controlled studies ranged from 600-1200 mg/day; some patients (8%) in the compassionate use program were treated with higher daily doses (1600-3200 mg/day). In the three-month controlled trials comparing MEXITIL to quinidine, procainamide and disopyramide, the most frequent adverse reactions were upper gastrointestinal distress (41%), lightheadedness (10.5%), tremor (12.6%) and coordination difficulties (10.2%). Similar frequency and incidence were observed in the one-month placebo-controlled trial. Although these reactions were generally not serious, and were dose-related and reversible with a reduction in dosage, by taking the drug with food or antacid or by therapy discontinuation, they led to therapy discontinuation in 40% of patients in the controlled trials. Table 1 presents the adverse events reported in the one-month placebo-controlled trial.
| Mexiletine
N=53 | Placebo
N=49 |
|
| Cardiovascular | ||
| Palpitations | 7.5 | 10.2 |
| Chest Pain | 7.5 | 4.1 |
| Increased Ventricular Arrhythmia /PVC's |
1.9 |
- |
| Digestive | ||
| Nausea/Vomiting/Heartburn | 39.6 | 6.1 |
| Central Nervous System | ||
| Dizziness/ Lightheadedness |
26.4 |
14.3 |
| Tremor | 13.2 | — |
| Nervousness | 11.3 | 6.1 |
| Coordination Difficulties | 9.4 | — |
| Changes in Sleep Habits | 7.5 | 16.3 |
| Paresthesias/Numbness | 3.8 | 2.0 |
| Weakness | 1.9 | 4.1 |
| Fatigue | 1.9 | 2.0 |
| Tinnitus | 1.9 | 4.1 |
| Confusion/Clouded Sensorium | 1.9 | 2.0 |
| Other | ||
| Headache | 7.5 | 6.1 |
| Blurred Vision/Visual Disturbances |
7.5 |
2.0 |
| Dyspnea/Respiratory | 5.7 | 10.2 |
| Rash | 3.8 | 2.0 |
| Non-specific Edema | 3.8 | — |
Table 2 presents the adverse reactions occurring in one percent or more of patients in the three-month controlled studies.
| Mexiletine | Quinidine | Procainamide | Disopyramide | |||||
| N = 430 | N = 262 | N = 78 | N = 69 | |||||
| Cardiovascular | ||||||||
| Palpitations | 4.3 | 4.6 | 1.3 | 5.8 | ||||
| Chest Pain | 2.6 | 3.4 | 1.3 | 2.9 | ||||
| Angina/Angina- like Pain |
1.7 |
1.9 |
2.6 |
2.9 |
||||
| Increased Ventricular Arrhythmias/PVC's |
1.0 |
2.7 |
2.6 |
— |
||||
| Digestive | ||||||||
| Nausea/Vomiting/Heartburn | 39.3 | 21.4 | 33.3 | 14.5 | ||||
| Diarrhea | 5.2 | 33.2 | 2.6 | 8.7 | ||||
| Constipation | 4.0 | — | 6.4 | 11.6 | ||||
| Changes in Appetite | 2.6 | 1.9 | — | — | ||||
| Abdominal Pain/Cramps/Discomfort | 1.2 | 1.5 | — | 1.4 | ||||
| Central Nervous System | ||||||||
| Dizziness/Lightheadedness | 18.9 | 14.1 | 14.1 | 2.9 | ||||
| Tremor | 13.2 | 2.3 | 3.8 | 1.4 | ||||
| Coordination Difficulties | 9.7 | 1.1 | 1.3 | — | ||||
| Changes in Sleep Habits | 7.1 | 2.7 | 11.5 | 8.7 | ||||
| Weakness | 5.0 | 5.3 | 7.7 | 2.9 | ||||
| Nervousness | 5.0 | 1.9 | 6.4 | 5.8 | ||||
| Fatigue | 3.8 | 5.7 | 5.1 | 1.4 | ||||
| Speech Difficulties | 2.6 | 0.4 | — | — | ||||
| Confusion/Clouded Sensorium | 2.6 | — | 3.8 | — | ||||
| Paresthesias/Numbness | 2.4 | 2.3 | 2.6 | — | ||||
| Tinnitus | 2.4 | 1.5 | — | — | ||||
| Depression | 2.4 | 1.1 | 1.3 | 1.4 | ||||
| Other | ||||||||
| Blurred Vision/Visual Disturbances | 5.7 | 3.1 | 5.1 | 7.2 | ||||
| Headache | 5.7 | 6.9 | 7.7 | 4.3 | ||||
| Rash | 4.2 | 3.8 | 10.3 | 1.4 | ||||
| Dyspnea/ Respiratory | 3.3 | 3.1 | 5.1 | 2.9 | ||||
| Dry Mouth | 2.8 | 1.9 | 5.1 | 14.5 | ||||
| Arthralgia | 1.7 | 2.3 | 5.1 | 1.4 | ||||
| Fever | 1.2 | 3.1 | 2.6 | — | ||||
Less than 1%: Syncope, edema, hot flashes, hypertension, short-term memory loss, loss of consciousness, other psychological changes, diaphoresis, urinary hesitancy/retention, malaise, impotence/decreased libido, pharyngitis, congestive heart failure.
An additional group of over 10,000 patients has been treated in a program allowing administration of MEXITIL (mexiletine hydrochloride, USP) under compassionate use circumstances. These patients were seriously ill with the large majority on multiple drug therapy. Twenty-four percent of the patients continued in the program for one year or longer. Adverse reactions leading to therapy discontinuation occurred in 15 percent of patients (usually upper gastrointestinal system or nervous system effects). In general, the more common adverse reactions were similar to those in the controlled trials. Less common adverse events possibly related to MEXITIL use include:
Cardiovascular System: Syncope and hypotension, each about 6 in 1000; bradycardia, about 4 in 1000; angina/angina-like pain, about 3 in 1000; edema, atrioventricular block/conduction disturbances and hot flashes, each about 2 in 1000; atrial arrhythmias, hypertension and cardiogenic shock, each about 1 in 1000.
Central Nervous System: Short-term memory loss, about 9 in 1000 patients; hallucinations and other psychological changes, each about 3 in 1000; psychosis and convulsions/seizures, each about 2 in 1000; loss of consciousness, about 6 in 10,000.
Digestive: Dysphagia, about 2 in 1000; peptic ulcer, about 8 in 10,000; upper gastrointestinal bleeding, about 7 in 10,000; esophageal ulceration, about 1 in 10,000. Rare cases of severe hepatitis/acute hepatic necrosis.
Skin: Rare cases of exfoliative dermatitis and Stevens-Johnson Syndrome with MEXITIL (mexiletine hydrochloride, USP) treatment have been reported.
Laboratory: Abnormal liver function tests, about 5 in 1000 patients; positive ANA and thrombocytopenia, each about 2 in 1000; leukopenia (including neutropenia and agranulocytosis), about 1 in 1000; myelofibrosis, about 2 in 10,000 patients.
Other: Diaphoresis, about 6 in 1000; altered taste, about 5 in 1000; salivary changes, hair loss and impotence/decreased libido, each about 4 in 1000; malaise, about 3 in 1000; urinary hesitancy/retention, each about 2 in 1000; hiccups, dry skin, laryngeal and pharyngeal changes and changes in oral mucous membranes, each about 1 in 1000; SLE syndrome, about 4 in 10,000.
Hematology
Blood dyscrasias were not seen in the controlled trials but did occur among 10,867 patients treated with mexiletine in the compassionate use program (see PRECAUTIONS).
Myelofibrosis was reported in two patients in the compassionate use program: one was receiving long-term thiotepa therapy and the other had pretreatment myeloid abnormalities.
In post-marketing experience, there have been isolated, spontaneous reports of pulmonary changes including pulmonary infiltration and pulmonary fibrosis during MEXITIL therapy with or without other drugs or diseases that are known to produce pulmonary toxicity. A causal relationship to MEXITIL therapy has not been established. In addition, there have been isolated reports of drowsiness, nystagmus, ataxia, dyspepsia, hypersensitivity reaction, and exacerbation of congestive heart failure in patients with pre-existing compromised ventricular function. There have been rare reports of pancreatitis associated with MEXITIL treatment.
Overdosage
Clinical findings associated with MEXITIL (mexiletine hydrochloride, USP) overdosage have included drowsiness, confusion, nausea, hypotension, sinus bradycardia, paresthesia, seizures, bundle branch block, AV heart block, asystole, ventricular tachyarrhythmia, including ventricular fibrillation, cardiovascular collapse and coma. The lowest known dose in a fatality case was 4.4g with postmortem serum mexiletine level of 34-37 mcg/ml (Jequier P. et al. Lancet 1976: 1 (7956): 429). Patients have recovered from ingestion of 4g to 18g of mexiletine (Frank S. E. et al. Am J Emerg Med 1991: 9:43-48).
There is no specific antidote for MEXITIL. Management of MEXITIL overdosage includes general supportive measures, close observation and monitoring of vital signs. In addition, the use of pharmacologic interventions (e.g., pressor agents, atropine or anticonvulsants) or transvenous cardiac pacing is suggested, depending on the patient's clinical condition.
Mexitil Dosage and Administration
The dosage of MEXITIL (mexiletine hydrochloride, USP) must be individualized on the basis of response and tolerance, both of which are dose-related. Administration with food or antacid is recommended. Initiate MEXITIL therapy with 200 mg every eight hours when rapid control of arrhythmia is not essential. A minimum of two to three days between dose adjustments is recommended. Dose may be adjusted in 50 or 100 mg increments up or down.
As with any antiarrhythmic drug, clinical and electrocardiographic evaluation (including Holter monitoring if necessary for evaluation) are needed to determine whether the desired antiarrhythmic effect has been obtained and to guide titration and dose adjustment.
Satisfactory control can be achieved in most patients by 200 to 300 mg given every eight hours with food or antacid. If satisfactory response has not been achieved at 300 mg q8h, and the patient tolerates MEXITIL well, a dose of 400 mg q8h may be tried. As the severity of CNS side effects increases with total daily dose, the dose should not exceed 1200 mg/day.
In general, patients with renal failure will require the usual doses of MEXITIL. Patients with severe liver disease, however, may require lower doses and must be monitored closely. Similarly, marked right-sided congestive heart failure can reduce hepatic metabolism and reduce the needed dose. Plasma level may also be affected by certain concomitant drugs (see PRECAUTIONS: Drug Interactions).
Loading Dose
When rapid control of ventricular arrhythmia is essential, an initial loading dose of 400 mg of MEXITIL may be administered, followed by a 200 mg dose in eight hours. Onset of therapeutic effect is usually observed within 30 minutes to two hours.
Q12H Dosage Schedule
Some patients responding to MEXITIL may be transferred to a 12-hour dosage schedule to improve convenience and compliance. If adequate suppression is achieved on a MEXITIL dose of 300 mg or less every eight hours, the same total daily dose may be given in divided doses every 12 hours while carefully monitoring the degree of suppression of ventricular ectopy. This dose may be adjusted up to a maximum of 450 mg every 12 hours to achieve the desired response.
Transferring to Mexitil
The following dosage schedule, based on theoretical considerations rather than experimental data, is suggested for transferring patients from other Class I oral antiarrhythmic agents to MEXITIL: MEXITIL treatment may be initiated with a 200 mg dose, and titrated to response as described above, 6-12 hours after the last dose of quinidine sulfate, 3-6 hours after the last dose of procainamide, 6-12 hours after the last dose of disopyramide or 8-12 hours after the last dose of tocainide.
In patients in whom withdrawal of the previous antiarrhythmic agent is likely to produce life-threatening arrhythmias, hospitalization of the patient is recommended.
When transferring from lidocaine to MEXITIL, the lidocaine infusion should be stopped when the first oral dose of MEXITIL is administered. The infusion line should be left open until suppression of the arrhythmia appears to be satisfactorily maintained. Consideration should be given to the similarity of the adverse effects of lidocaine and MEXITIL and the possibility that they may be additive.
How is Mexitil supplied
MEXITIL (mexiletine hydrochloride, USP) is supplied in bottles of 100 hard gelatin capsules containing 150 mg, 200 mg or 250 mg of mexiletine hydrochloride:
MEXITIL 150 mg capsules are red and caramel with the marking Bl 66 (NDC 0597-0066-01).
MEXITIL 200 mg capsules are red with the marking Bl 67 (NDC 0597-0067-01).
MEXITIL 250 mg capsules are red and aqua green with the marking Bl 68 (NDC 0597-0068-01).
Store at 25°C (77°F); excursions permitted to 15-30°C (59-86°F). [see USP Controlled Room Temperature].
Rx Only
Distributed by;
Boehringer Ingelheim
Pharmaceuticals, Inc.
Ridgefield, Connecticut 06877 USA
Licensed from;
Boehringer Ingelheim
International GmbH
© Copyright Boehringer Ingelheim International GmbH
2003, ALL RIGHTS RESERVED
05/30/03
4056755/US/2
4056755//2
| MEXITIL
mexiletine hydrochloride capsule |
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| MEXITIL
mexiletine hydrochloride capsule |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| MEXITIL
mexiletine hydrochloride capsule |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Labeler - Boehringer Ingelheim Pharmaceuticals, Inc. |
More about Mexitil (mexiletine)
- Check interactions
- Compare alternatives
- Reviews (3)
- Drug images
- Side effects
- Dosage information
- During pregnancy
- Drug class: group I antiarrhythmics
- Breastfeeding